Molecular modelling of human CYP2D6 and molecular docking of a series of ajmalicine- and quinidine-like inhibitors.
Marilena Saraceno, Alessio Coi, Anna Maria Bianucci
Index: Int. J. Biol. Macromol. 42(4) , 362-71, (2008)
Full Text: HTML
Abstract
3D-models were created and refined for CYP2D6 and for its complexes with ajmalicine and quinidine. The influence of the conformation of the enzyme active site on its interaction with ligands was evaluated by performing three series of molecular docking on selected ajmalicine- and quinidine-like inhibitors. The results suggested that the experimental binding values of ajmalicine- and quinidine-like inhibitors better fit with the energetic terms derived from their interaction with structures of CYP2D6 obtained by, respectively, optimizing the ajmalicine/CYP2D6 and the quinidine/CYP2D6 complexes, rather than exploiting the 3D-strucure of the enzyme not subjected to a ligand-induced conformational change. It suggests the relevance of induced-fit phenomena in the biological system of interest.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
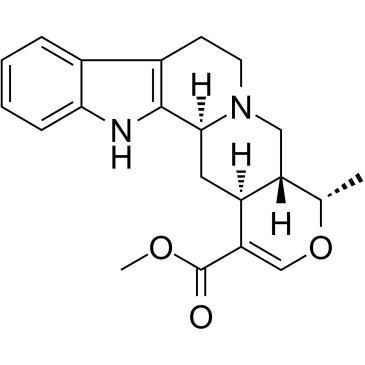 |
Ajmalicine
CAS:483-04-5 |
C21H24N2O3 |
|
Determination of terpenoid indole alkaloids in hairy roots o...
2015-01-01 [Phytochem. Anal. 26 , 331-8, (2015)] |
|
Synergistic and cytotoxic action of indole alkaloids produce...
2013-03-01 [Pharm. Biol. 51(3) , 304-10, (2013)] |
|
A differential response to chemical elicitors in Catharanthu...
2009-04-01 [Biotechnol. Lett. 31(4) , 591-5, (2009)] |
|
Construction and expression of a dual vector for chemo-enzym...
2010-05-01 [Nat. Prod. Res. 24(8) , 759-66, (2010)] |
|
Quantitative determination of reserpine, ajmaline, and ajmal...
2006-10-01 [J. Chromatogr. Sci. 44(9) , 557-60, (2006)] |