Photostability and photoprotection factor of boldine and glaucine.
María Eliana Hidalgo, Miguel Farah, Leonardo Carrasco, Ernesto Fernández
Index: J. Photochem. Photobiol. B, Biol. 80(1) , 65-9, (2005)
Full Text: HTML
Abstract
Boldine hydrochloride was more photounstable than boldine after irradiation with UVB (lambda = 300 nm). However, photoconsumption quantum yields, for glaucine hydrochloride (6.5 x 10(-2)) and boldine hydrochloride (6.7 x 10(-2)) in air, were quite similar. The photolysis was oxygen dependent in both cases, and the effect over the kinetics after the addition of 2,2,6,6-tetramethyl-1-piperidinyloxy suggested free radicals participation. The fact that the antioxidative capacity of boldine and boldine hydrochloride did not change during the photolysis, suggests that the phenolic structure remains unchanged in the photoproducts, corroborated with the photoproducts analysis. The photoprotection capacity was evaluated before and after irradiation. Results indicate that the values before irradiation are similar for all three compounds, only glaucine increasing its capacity with length of irradiation time.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
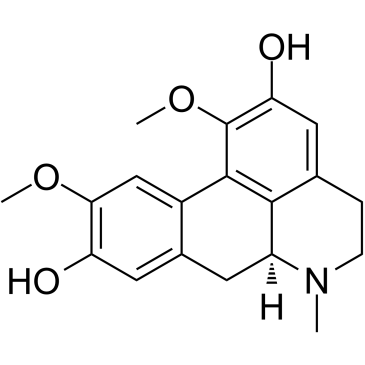 |
Boldine
CAS:476-70-0 |
C19H21NO4 |
|
Applied biological and physicochemical activity of isoquinol...
2012-01-01 [Molecules 17(9) , 10958-70, (2012)] |
|
Are extraction methods in quantitative assays of pharmacopoe...
2006-10-01 [Planta Med. 72(12) , 1157-62, (2006)] |
|
8-NH2-boldine, an antagonist of alpha1A and alpha1B adrenoce...
2005-10-01 [Planta Med. 71(10) , 897-903, (2005)] |
|
The aporphine alkaloid boldine induces adiponectin expressio...
2009-10-01 [J. Med. Food 12(5) , 1074-83, (2009)] |
|
Aporphine metho salts as neuronal nicotinic acetylcholine re...
2007-05-15 [Bioorg. Med. Chem. 15(10) , 3368-72, (2007)] |