Preparation and characterization of 8a-(phosphatidylcholine-dioxy)-alpha-tocopherones and their formation during the peroxidation of phosphatidylcholine in liposomes.
R Yamauchi, H Mizuno, K Kato
Index: Biosci. Biotechnol. Biochem. 62(7) , 1293-300, (1998)
Full Text: HTML
Abstract
alpha-Tocopherol was reacted with the phosphatidylcholines (PCs), 1-palmitoyl-2-linoleoyl-3-sn-PC (PLPC), 1-palmitoyl-2-linolenoyl-3-sn-PC, 1-palmitoyl-2-arachidonoyl-3-sn-PC (PAPC) and 1-stearoyl-2-arachidonoyl-3-sn-PC, in the presence of the free radical initiator, 2,2'-azobis (2,4-dimethylvaleronitrile), at 37 degrees C. The addition products of alpha-tocopherol with the PC peroxyl radicals were isolated and identified as 8a-(PC-dioxy)-alpha-tocopherones, in which the peroxyl radicals derived from each PC molecule attacked the 8a-position of the alpha-tocopheroxyl radical. The antioxidative efficiency of alpha-tocopherol against the peroxidation of PLPC and PAPC in liposomes was assessed by the formation of the reaction products of alpha-tocopherol. When alpha-tocopherol was oxidized in the presence of the water-soluble free radical initiator, 2,2'-azobis (2-amidinopropane) dihydrochloride, epoxy-alpha-tocopherylquinones were mainly produced together with 8a-(PC-dioxy)-alpha-tocopherones and alpha-tocopherylquinone. The yield of alpha-tocopherylquinone was increased by treating each sample with dilute acid which indicates the presence of tocopherone precursors other than the 8a-(PC-dioxy)-alpha-tocopherones. The same products were also detected from iron-dependent peroxidation, although the yields were very low.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
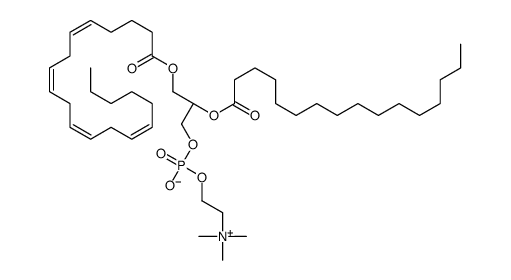 |
1-Palmitoyl-2-Arachidonoyl-sn-glycero-3-PC
CAS:35418-58-7 |
C44H80NO8P |
|
Miscibility of Sphingomyelins and Phosphatidylcholines in Un...
2015-11-03 [Biophys. J. 109 , 1907-16, (2015)] |
|
CTP:phosphocholine cytidylyltransferase activation by oxidiz...
1999-11-23 [Biochemistry 38(47) , 15606-14, (1999)] |
|
Characterization of hormonally regulated and particulate-ass...
1993-06-05 [J. Biol. Chem. 268(16) , 11697-702, (1993)] |
|
Transfer of very low density lipoprotein-associated phosphol...
2006-02-01 [J. Lipid Res. 47(2) , 341-8, (2006)] |
|
A novel phospholipase A2 activity in saliva of the lone star...
1997-10-01 [Exp. Parasitol. 87(2) , 121-32, (1997)] |