Catalytic enantioselective protonation of nitronates utilizing an organocatalyst chiral only at sulfur.
Kyle L Kimmel, Jimmie D Weaver, Melissa Lee, Jonathan A Ellman
Index: J. Am. Chem. Soc. 134(22) , 9058-61, (2012)
Full Text: HTML
Abstract
The highly enantioselective protonation of nitronates formed upon the addition of α-substituted Meldrum's acids to terminally unsubstituted nitroalkenes is described. This work represents the first enantioselective catalytic addition of any type of nucleophile to this class of nitroalkenes. Moreover, for the successful implementation of this method, a new type of N-sulfinyl urea catalyst with chirality residing only at the sulfinyl group was developed, thereby enabling the incorporation of a diverse range of achiral diamine motifs. Finally, the Meldrum's acid addition products were readily converted to pharmaceutically relevant 3,5-disubstituted pyrrolidinones in high yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
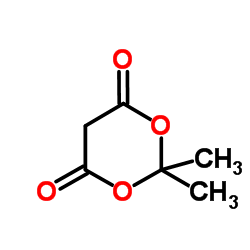 |
Meldrumic acid
CAS:2033-24-1 |
C6H8O4 |
|
Synthesis and biological evaluation of arylidene analogues o...
2010-08-01 [Bioorg. Med. Chem. 18 , 5626-33, (2010)] |
|
One hundred years of Meldrum's acid: advances in the synthes...
2009-11-01 [Mol. Divers. 13(4) , 399-419, (2009)] |
|
A new domino autocatalytic reaction leading to polyfunctiona...
2009-05-21 [Org. Biomol. Chem. 7(10) , 2195-201, (2009)] |
|
A novel one-pot three-(in situ five-)component condensation ...
2009-08-06 [Org. Lett. 11(15) , 3342-5, (2009)] |
|
Self-assembled Pd6 open cage with triimidazole walls and the...
2012-09-24 [Chemistry 18(39) , 12322-9, (2012)] |