| Structure | Name/CAS No. | Articles |
|---|---|---|
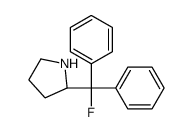 |
(S)-(-)-2-(FLUORODIPHENYLMETHYL)PYRROLI&
CAS:274674-23-6 |
|
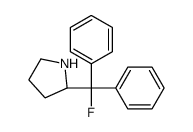 |
(R)-(+)-2-(FLUORODIPHENYLMETHYL)PYRROLI&
CAS:352535-00-3 |