Calculations of the energy distribution from perturbation peak data—A new tool for characterization of chromatographic phases
Jörgen Samuelsson, Torgny Fornstedt, Jörgen Samuelsson, Torgny Fornstedt
Index: J. Chromatogr. A. 1203(2) , 177-84, (2008)
Full Text: HTML
Abstract
The calculation of the adsorption energy distribution (AED) was recently introduced as an important tool for the chromatographic community for characterization of modern phases. The AED-calculations, provides model-independent information about the numbers of different adsorption sites and their respective energy-levels, prior to the selection of an adsorption isotherm model which narrows the number of possible rival models. The selection of a proper model for the fitting of the determined raw data is crucial; if the wrong model is selected misleading information about the retention mechanism may be drawn. The AED-calculations require raw adsorption isotherm data (i.e. data points) which is unfortunately not obtained by the newly validated perturbation peak method. In this study, we developed mathematical expression allowing the use of the raw tangential slope provided by the perturbation peak method for AED calculations. The approach worked excellently and was verified against both computer-generated adsorption isotherm data as well as experimentally determined data, using three different experimental systems. It was found that the calculations of the AED, as based on perturbation peak data, converts faster and are not more sensitive to experimental noise as compared to the classical AED calculations using raw adsorption isotherm data.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
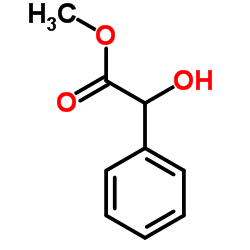 |
(±)-methyl mandelate
CAS:4358-87-6 |
C9H10O3 |
|
Hydrolysis of triacetin catalyzed by immobilized lipases: Ef...
2011-01-01 [Enzyme Microb. Technol. 48 , 510-517, (2011)] |
|
Esters of mandelic acid as substrates for (S)-mandelate dehy...
2004-02-24 [Biochemistry 43(7) , 1883-90, (2004)] |
|
Generation of the enol of methyl mandelate by flash photolys...
2000-02-25 [J. Org. Chem. 65(4) , 1175-80, (2000)] |
|
Theoretical study on chiral recognition mechanism of methyl ...
2012-02-01 [J. Mol. Model. 18(2) , 803-13, (2012)] |
|
Compensation of the enantioselectivity-activity trade-off in...
2013-04-01 [Appl. Microbiol. Biotechnol. 97(8) , 3355-62, (2013)] |