Generation of the enol of methyl mandelate by flash photolysis of methyl phenyldiazoacetate in aqueous solution and study of rates of ketonization of this enol in that medium.
Y Chiang, A J Kresge, N P Schepp, R Xie
Index: J. Org. Chem. 65(4) , 1175-80, (2000)
Full Text: HTML
Abstract
Flash photolysis of methyl phenyldiazoacetate in aqueous solution produced phenylcarbomethoxycarbene, whose hydration generated a short-lived transient species that was identified as the enol isomer of methyl mandelate. This assignment is supported by the shape of the rate profile for decay of the enol transient, through ketonization to its carbonyl isomer, as well as by solvent isotope effects and the form of acid-base catalysis of the ketonization reaction. Comparison of the present results with previously published information on the enol of mandelic acid shows some interesting and readily understandable similarities and differences.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
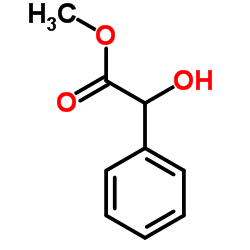 |
(±)-methyl mandelate
CAS:4358-87-6 |
C9H10O3 |
|
Hydrolysis of triacetin catalyzed by immobilized lipases: Ef...
2011-01-01 [Enzyme Microb. Technol. 48 , 510-517, (2011)] |
|
Esters of mandelic acid as substrates for (S)-mandelate dehy...
2004-02-24 [Biochemistry 43(7) , 1883-90, (2004)] |
|
Theoretical study on chiral recognition mechanism of methyl ...
2012-02-01 [J. Mol. Model. 18(2) , 803-13, (2012)] |
|
Calculations of the energy distribution from perturbation pe...
2008-01-01 [J. Chromatogr. A. 1203(2) , 177-84, (2008)] |
|
Compensation of the enantioselectivity-activity trade-off in...
2013-04-01 [Appl. Microbiol. Biotechnol. 97(8) , 3355-62, (2013)] |