EORTC trial 11001: distribution of two 10B-compounds in patients with squamous cell carcinoma of head and neck, a translational research/phase 1 trial.
Andrea Wittig, Laurence Collette, Klaas Appelman, Sandra Bührmann, Martin C Jäckel, Karl-Heinz Jöckel, Kurt Werner Schmid, Uta Ortmann, Raymond Moss, Wolfgang A G Sauerwein
Index: J. Cell. Mol. Med. 13(8B) , 1653-65, (2009)
Full Text: HTML
Abstract
Boron neutron capture therapy (BNCT) provides highly targeted delivery of radiation through the limited spatial distribution of its effects. This translational research/phase I clinical trial investigates whether BNCT might be developed as a treatment option for squamous cell carcinoma of head and neck (SCCHN) relying upon preferential uptake of the two compounds, sodium mercaptoundecahydro-closo-dodecaborate (BSH) or L-para-boronophenylalanine (BPA) in the tumour. Before planned tumour resection, three patients received BSH and three patients received BPA. The (10)B-concentration in tissues and blood was measured with prompt gamma ray spectroscopy. Adverse effects from compounds did not occur. After BPA infusion the (10)B-concentration ratio of tumour/blood was 4.0 +/- 1.7. (10)B-concentration ratios of tumour/normal tissue were 1.3 +/- 0.5 for skin, 2.1 +/- 1.2 for muscle and 1.4 +/- 0.01 for mucosa. After BSH infusion the (10)B-concentration ratio of tumour/blood was 1.2 +/- 0.4. (10)B-concentration ratios of tumour/normal tissue were 3.6 +/- 0.6 for muscle, 2.5 +/- 1.0 for lymph nodes, 1.4 +/- 0.5 for skin and 1.0 +/- 0.3 for mucosa. BPA and BSH deliver (10)B to SCCHN to an extent that might allow effective BNCT treatment. Mucosa and skin are the most relevant organs at risk.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
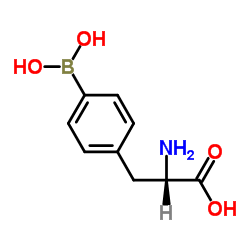 |
4-Borono-L-phenylalanine
CAS:76410-58-7 |
C9H12BNO4 |
|
Pharmacokinetic analysis and uptake of 18F-FBPA-Fr after ult...
2014-04-01 [J. Nucl. Med. 55(4) , 616-21, (2014)] |
|
Low dose of gamma irradiation enhanced boronophenylalanine u...
2011-12-01 [Appl. Radiat. Isot. 69(12) , 1728-31, (2011)] |
|
Boron neutron capture therapy induces apoptosis of glioma ce...
2010-01-01 [BMC Cancer 10 , 661, (2010)] |
|
Studies for the application of boron neutron capture therapy...
2011-12-01 [Appl. Radiat. Isot. 69(12) , 1752-5, (2011)] |
|
Boron neutron capture therapy for clear cell sarcoma (CCS): ...
2011-12-01 [Appl. Radiat. Isot. 69(12) , 1721-4, (2011)] |