Type-2 isopentenyl diphosphate isomerase: evidence for a stepwise mechanism.
Nicole A Heaps, C Dale Poulter
Index: J. Am. Chem. Soc. 133 , 19017-19019, (2011)
Full Text: HTML
Abstract
Isopentenyl diphosphate isomerase (IDI) catalyzes the interconversion of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These two molecules are the building blocks for construction of isoprenoid carbon skeletons in nature. Two structurally unrelated forms of IDI are known. A variety of studies support a proton addition/proton elimination mechanism for both enzymes. During studies with Thermus thermophilus IDI-2, we discovered that the olefinic hydrogens of a vinyl thiomethyl analogue of isopentenyl diphosphate exchanged with solvent when the enzyme was incubated with D(2)O without concomitant isomerization of the double bond. These results suggest that the enzyme-catalyzed isomerization reaction is not concerted.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
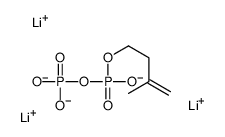 |
Isopentenyl pyrophosphate trilithium salt
CAS:18687-43-9 |
C5H9Li3O7P2 |
|
An improved synthesis of isopentenyl pyrophosphate.
1967-11-01 [Biochem. J. 105 , 545-547, (1967)] |
|
The biosynthesis of rubber. Incorporation of mevalonate and ...
1963-12-01 [Biochem. J. 89(3) , 565-74, (1963)] |
|
The biosynthesis of isoprenoids and the mechanisms regulatin...
2011-01-01 [Biosci. Biotechnol. Biochem. 75 , 1219-1225, (2011)] |
|
Type II isopentenyl diphosphate isomerase: probing the mecha...
2010-07-27 [Biochemistry 49 , 6228-6233, (2010)] |
|
Isopentenyl diphosphate isomerase catalyzed reactions in D2O...
2012-04-18 [J. Am. Chem. Soc. 134 , 6568-6570, (2012)] |