Photophysical properties of 3,3'-dialkylthiacarbocyanine dyes in organized media: unilamellar liposomes and thin polymer films.
M Krieg, M B Srichai, R W Redmond
Index: Biochim. Biophys. Acta 1151 , 168, (1993)
Full Text: HTML
Abstract
All symmetrical dialkylthiacarbocyanine dyes, with the exception of the diethyl derivatives, are incorporated into liposomes. Absorption and fluorescence data indicate a solubilization site close to the bilayer surface with the alkyl chains penetrating into the lipid bilayer. Incorporation into organized assemblies affects the photophysical parameters of these dyes. Photoisomerization occurring from the first excited state becomes more difficult as the restrictive effect of the solubilization site increases. As a consequence, competing deactivation processes, such as fluorescence and triplet formation, become more efficient with the result that fluorescence quantum yields, triplet yields and singlet oxygen quantum yields are larger in liposomes than in homogeneous solution. Dihexylthiacarbocyanine iodide has a fluorescence quantum yield of 0.27 and 0.10 (25 degrees C) in dimyristoylphosphatidyl-choline liposomes and ethanol, respectively, and the singlet oxygen yield increases by a factor three to 0.006 on going from ethanol to liposomes. The effect of a highly organized environment is even more pronounced in thin polymer films. In these systems, photoisomerization is completely inhibited and only triplet formation is observed in the transient absorption spectrum.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
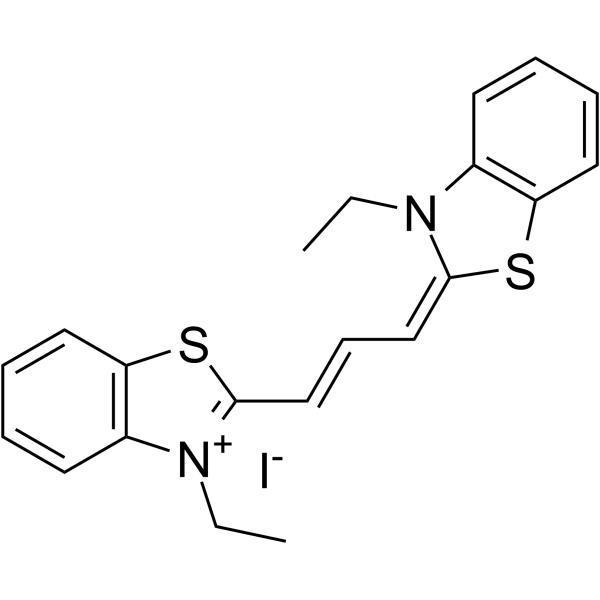 |
3,3'-Diethylthiacarbocyanine iodide
CAS:905-97-5 |
C21H21IN2S2 |
|
Structure-activity relationship of cyanine tau aggregation i...
2009-06-11 [J. Med. Chem. 52 , 3539-47, (2009)] |
|
In silico activity profiling reveals the mechanism of action...
2008-07-01 [Proc. Natl. Acad. Sci. U. S. A. 105 , 9059-64, (2008)] |
|
Three-dimensional fluorescence lifetime tomography.
2005-04-01 [Med. Phys. 32(4) , 992-1000, (2005)] |
|
Genetic screening using the colour change of a PNA-DNA hybri...
2002-01-15 [Nucleic Acids Res. 30(2) , E3, (2002)] |
|
Leucine transport is affected by Bacillus thuringiensis Cry1...
2006-01-01 [J. Membr. Biol. 214(3) , 157-64, (2006)] |