Expanding cavitand chemistry: the preparation and characterization of [n]cavitands with n>=4.
C Naumann, E Román, C Peinador, T Ren, B O Patrick, A E Kaifer, J C Sherman
Index: Chemistry 7(8) , 1637-45, (2001)
Full Text: HTML
Abstract
The preparation of cavitands composed of 4, 5, 6, and 7 aromatic subunits ([n]cavitands, n=4-7) is described. The simple, two-step synthetic procedure utilized readily available starting materials (2-methylresorcinol and diethoxymethane). The two cavitand products having 4 and 5 aromatic subunits exhibited highly symmetric cone conformations, while the larger cavitands (n = 6 and 7) adopt conformations of lower symmetry. 1H NMR spectroscopic studies of [6]cavitand and [7]cavitand revealed that these hosts undergo exchange between equivalent conformations at room temperature. The departure of these two cavitands from cone conformations is related to steric crowding on their Ar-O-CH2-OAr bridges and is predicted by simple molecular mechanics calculations (MM2 force field). X-ray diffraction studies on single crystals of the [4]cavitand, [5]cavitand, and [6]cavitand hosts afforded additional experimental support for these conclusions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
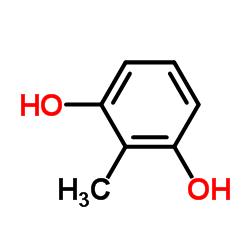 |
2-methylresorcinol
CAS:608-25-3 |
C7H8O2 |
|
Cross-elicitation responses to 2-methoxymethyl-p-phenylenedi...
2015-04-01 [Br. J. Dermatol. 172(4) , 976-80, (2015)] |
|
[Long-term preservation of DNA in aqueous solutions in the p...
2006-01-01 [Mikrobiologiia 75(5) , 662-9, (2006)] |
|
[Influence of chemical analogues of microbial autoregulators...
2006-01-01 [Mikrobiologiia 75(5) , 654-61, (2006)] |
|
A subchronic, teratologic, and dominant lethal study of 2-me...
1986-08-01 [Fundam. Appl. Toxicol. 7(2) , 293-8, (1986)] |
|
A subchronic, teratologic, and dominant lethal study of 2-me...
1986-08-01 [Fundam. Appl. Toxicol. 7(2) , 287-92, (1986)] |