| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(+/-)-butaclamol hcl
CAS:36504-94-6 |
|
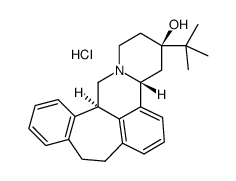 |
(-)-Butaclamol Hydrochloride
CAS:55528-08-0 |
|
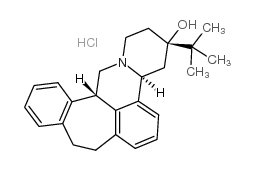 |
(+)-Butaclamol Hydrochloride
CAS:55528-07-9 |