| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
1-METHOXY-2-NAPHTHOIC ACID
CAS:883-21-6 |
|
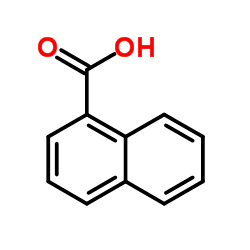 |
naphthoic acid
CAS:86-55-5 |
|
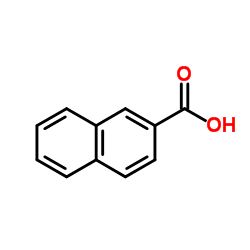 |
2-Naphthoic acid
CAS:93-09-4 |