Polypyrroles as antioxidants: kinetic studies on reactions of bilirubin and biliverdin dimethyl esters and synthetic model compounds with peroxyl radicals in solution. Chemical calculations on selected typical structures.
Leonid L Chepelev, Cory S Beshara, Patricia D MacLean, Gillian L Hatfield, Amy A Rand, Alison Thompson, James S Wright, L Ross C Barclay
Index: J. Org. Chem. 71(1) , 22-30, (2006)
Full Text: HTML
Abstract
[reaction: see text] Rate constants for hydrogen-atom transfer (HAT) from bilirubin dimethyl ester (BRDE) and biliverdin dimethyl ester (BVDE) to peroxyl radicals during inhibited autoxidation of styrene initiated by azo-bisisobutyronitrile (AIBN) were k(inh)(BRDE) = 22.5 x 10(4) and k(inh)(BVDE) = 10.2 x 10(4) M(-1) s(-1), and the stoichiometric factors (n) were 2.0 and 2.7, respectively. A synthetic tetrapyrrole (bis(dipyrromethene)) containing the alpha-central (2,2') CH2 linkage gave k(inh) = 39.9 x 10(4) M(-1) s(-1) and n = 2.3, whereas the beta-linked (3,3') isomer was not an active antioxidant. Several dipyrrinones were synthesized as mimics of the two outer heterocyclic rings of bilirubin and biliverdin. The dipyrrinones containing N-H groups in each ring were active antioxidants, whereas those lacking two such "free" N-H groups, such as N-CH3 dipyrrinones and dipyrromethenes, did not exhibit antioxidant activity. Overall, the relative k(inh) values compared to those of phenolic antioxidants, 2,6-di-tert-butyl-4-methoxyphenol (DBHA) and 2,6-di-tert-butyl-4-methylphenol (BHT), were 2,2'-bis(dipyrromethene) > BRDE > DBHA > dipyrrinones > BVDE > BHT. This general trend in antioxidant activities was also observed for the inhibited autoxidation of cumene initiated by AIBN. Chemical calculations of the N-H bond dissociation enthalpies (BDEs) of the typical structures support a HAT mechanism from N-H groups to trap peroxyl radicals. Intramolecular hydrogen bonding of intermediate nitrogen radicals has a major influence on the antioxidant activities of all compounds studied. Indeed, chemical calculations showed that the initial nitrogen radical from a dipyrrinone is stabilized by 9.0 kcal/mol because of H-bonding between the N-H remaining on one ring and the ground-state pyrrolyl radical of the adjacent ring in the natural zusammen structure. The calculated minimum structure of bilirubin shows strong intramolecular H-bonding of the N-H groups with carbonyl groups resulting in the known "ridge-tile" structure which is not an active HAT antioxidant. The calculated minimum structure of biliverdin is planar. BRDE is readily converted into BVDE by reaction with the electron-deficient DPPH* radical under argon in chlorobenzene. An electron-transfer mechanism is proposed for the initiating step in this reaction, and this is supported by the relatively low ionizing potential of a model dipyrrole representing the two central rings of bilirubin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
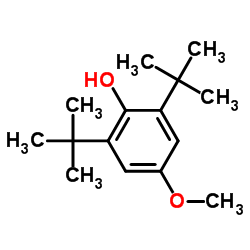 |
3,5-di-t-Butyl-4-hydroxyanisole
CAS:489-01-0 |
C15H24O2 |
|
Structural characterization of lignin: a potential source of...
2015-04-01 [Int. J. Biol. Macromol. 75 , 58-66, (2015)] |
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a...
2005-11-17 [J. Med. Chem. 48 , 7234-42, (2005)] |
|
Discovery of novel SERCA inhibitors by virtual screening of ...
2011-05-01 [Eur. J. Med. Chem. 46 , 1512-23, (2011)] |
|
Carbonic anhydrase inhibitors. Inhibition of human erythrocy...
2009-04-15 [Bioorg. Med. Chem. 17 , 3207-11, (2009)] |
|
In(OTf)3-catalyzed tandem nucleophilic addition and cyclizat...
2006-06-02 [Angew. Chem. Int. Ed. Engl. 45(23) , 3822-5, (2006)] |