| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Propofol
CAS:2078-54-8 |
|
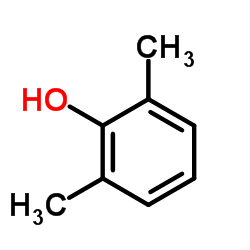 |
2,6-Xylenol
CAS:576-26-1 |
|
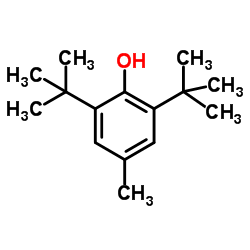 |
Butylated hydroxytoluene
CAS:128-37-0 |
|
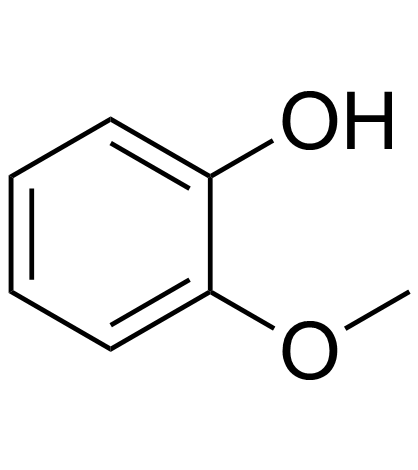 |
Guaiacol
CAS:90-05-1 |
|
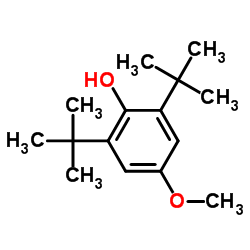 |
3,5-di-t-Butyl-4-hydroxyanisole
CAS:489-01-0 |
|
 |
2,6-Di-tert-butylphenol
CAS:128-39-2 |