Structures of mutagens produced by the co-mutagen norharman with o- and m-toluidine isomers.
N Hada, Y Totsuka, T Enya, K Tsurumaki, M Nakazawa, N Kawahara, Y Murakami, Y Yokoyama, T Sugimura, K Wakabayashi
Index: Mutat. Res. 493(1-2) , 115-26, (2001)
Full Text: HTML
Abstract
Norharman, abundantly present in cigarette smoke and cooked foods, is not mutagenic to Salmonella typhimurium strains. However, norharman shows mutagenicity to S. typhimurium TA98 and YG1024 in the presence of S9 mix when coexisting with aromatic amines, including aniline, o- and m-toluidines. We previously reported that the mutagenicity from norharman and aniline in the presence of S9 mix was due to the formation of a mutagenic compound, 9-(4'-aminophenyl)-9H-pyrido[3,4-b]indole (aminophenylnorharman). In the present study, we analyzed the mutagens produced by norharman with o- or m-toluidine in the presence of S9 mix. When norharman and o-toluidine were reacted at 37 degrees C for 20 min, two mutagenic compounds, which were mutagenic with and without S9 mix, respectively, were produced, and these were isolated by HPLC. The former mutagen was deduced to be 9-(4'-amino-3'-methylphenyl)-9H-pyrido[3,4-b]indole (amino-3'-methylphenylnorharman) on the basis of various spectral data, and this new heterocyclic amine was confirmed by its chemical synthesis. The latter mutagen was identified to be the hydroxyamino derivative. Amino-3'-methylphenylnorharman induced 41,000 revertants of TA98, and 698,000 revertants of YG1024 per microg with S9 mix. Formation of the same DNA adducts was observed in YG1024 when amino-3'-methylphenylnorharman or a mixture of norharman plus o-toluidine was incubated with S9 mix. These observations suggest that norharman reacts with o-toluidine in the presence of S9 mix to produce amino-3'-methylphenylnorharman, and this compound is metabolically activated to yield its hydroxyamino derivative. After activation by O-acetyltransferase, it might bind to DNA and exert mutagenicity in S. typhimurium TA98 and YG1024. When norharman and m-toluidine were reacted in the presence of S9 mix, 9-(4'-amino-2'-methylphenyl)-9H-pyrido[3,4-b]indole (amino-2'-methylphenylnorharman) was identified as a mutagen. Thus, the mutagenicity of norharman with m-toluidine may follow a mechanism similar to that with o-toluidine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
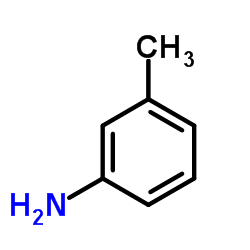 |
3-Toluidine
CAS:108-44-1 |
C7H9N |
|
cIEF for rapid pKa determination of small molecules: a proof...
2014-10-15 [Eur. J. Pharm. Sci. 63 , 14-21, (2014)] |
|
Optimizations of packed sorbent and inlet temperature for la...
2014-08-22 [J. Chromatogr. A. 1356 , 221-9, (2014)] |
|
Exposure of hairdressers to ortho- and meta-toluidine in hai...
2015-01-01 [Occup. Environ. Med. 72(1) , 57-63, (2015)] |
|
Effect of toluidines on drug metabolizing enzymes in rat liv...
1984-09-28 [Toxicology 32(4) , 335-42, (1984)] |
|
Biological monitoring of phenmedipham: determination of m-to...
2001-05-01 [Arch. Toxicol. 75(3) , 145-9, (2001)] |