| Structure | Name/CAS No. | Articles |
|---|---|---|
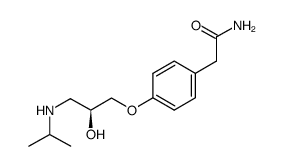 |
esatenolol
CAS:93379-54-5 |
|
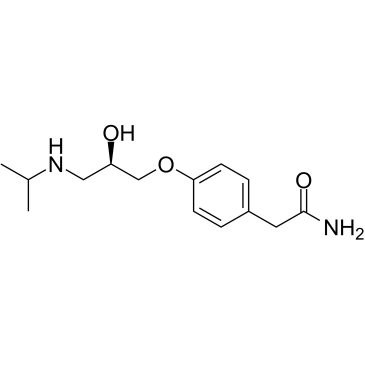 |
(R)-(+)-Atenolol
CAS:56715-13-0 |