Geminate recombination of n-butyl isocyanide to myoglobin.
J H Sommer, E R Henry, J Hofrichter
Index: Biochemistry 24(25) , 7380-8, (1985)
Full Text: HTML
Abstract
Transient optical absorption spectra of myoglobin were measured following photolysis of the n-butyl isocyanide complex with 10-ns laser pulses at room temperature. The data were analyzed by using singular value decomposition to give the kinetics of ligand rebinding and spectral changes. Geminate recombination phases were observed at 30 ns and 1 microsecond following photodissociation. These processes were accompanied by simultaneous changes in the shape of the Soret band which indicate changes in protein conformation. These spectral changes are not present in the geminate recombination of photolyzed complexes of myoglobin with the diatomic ligands oxygen and carbon monoxide. This difference in behavior, as well as the slower overall association rate of n-butyl isocyanide to myoglobin, can be rationalized as arising from distortion of the protein structure by the larger isocyanide ligand along the binding pathway.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
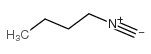 |
N-butylisocyanide
CAS:2769-64-4 |
C5H9N |
|
n-Butyl isocyanide oxidation at the [NiFe4S4OH(x)] cluster o...
2012-02-01 [J. Biol. Inorg. Chem. 17(2) , 167-73, (2012)] |
|
Butyl isocyanide as a probe of the activation mechanism of s...
2007-12-07 [J. Biol. Chem. 282(49) , 35741-8, (2007)] |
|
Kinetics of intramolecular charge transfer with N-phenylpyrr...
2005-03-03 [J. Phys. Chem. A 109(8) , 1497-509, (2005)] |
|
Alkyl and aromatic isocyanide binding to haem complexes.
1989-09-15 [Biochem. J. 262(3) , 959-63, (1989)] |
|
Proton nuclear magnetic resonance study of the binary comple...
1999-07-23 [FEBS Lett. 455(3) , 302-6, (1999)] |