Organic Letters
2007-04-12
Highly efficient ruthenium catalysts for the formation of tetrasubstituted olefins via ring-closing metathesis.
Ian C Stewart, Thay Ung, Alexandre A Pletnev, Jacob M Berlin, Robert H Grubbs, Yann Schrodi
Index: Org. Lett. 9 , 1589, (2007)
Full Text: HTML
Abstract
[reaction: see text] A series of ruthenium-based metathesis catalysts with N-heterocyclic carbene (NHC) ligands have been prepared in which the N-aryl groups have been changed from mesityl to mono-ortho-substituted phenyl (e.g., tolyl). These new catalysts offer an exceptional increase in activity for the formation of tetrasubstituted olefins via ring-closing metathesis (RCM), while maintaining high levels of activity in ring-closing metathesis (RCM) reactions that generate di- and trisubstituted olefins.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
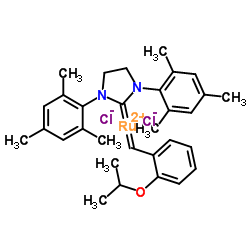 |
Hoveyda-Grubbs II
CAS:301224-40-8 |
C31H38Cl2N2ORu |
Related Articles:
More...
|
Sequential catalysis: a metathesis/dihydroxylation sequence.
2006-03-13 [Angew. Chem. Int. Ed. Engl. 45 , 1900, (2006)] |
|
Ruthenium catalysed cross metathesis with fluorinated olefin...
2001-09-07 [Chem. Commun. (Camb.) , 1692, (2001)] |
|
Ring-opening metathesis/oxy-cope rearrangement: a new strate...
2003-12-03 [J. Am. Chem. Soc. 125 , 14901, (2003)] |
|
Schrodi, Y.; Pederson, R. L.
[Aldrichimica Acta 40th ed.,, 45, (2007)] |
|
Vougioukalakis, G. C.; Grubbs, R. H.
[Chem. Rev. 110th ed.,, 1746, (2010)] |