Functional residues at the active site of horse liver phosphopantothenoylcysteine decarboxylase.
R Scandurra, V Consalvi, L Politi, C Gallina
Index: FEBS Lett. 231(1) , 192-6, (1988)
Full Text: HTML
Abstract
Horse liver phosphopantothenoylcysteine decarboxylase (EC 4.1.1.36) is rapidly inactivated by N-acetoacetylation with diketene following a pseudo-first-order kinetics: the presence of substrate quantitatively protects against this inactivation. Histidine photo-oxidation with methylene blue or rose bengal brings about the total loss of activity. These results indicate the presence of functional lysyl and histidyl groups at the active site of the enzyme. The substrate sulphydryl group is essential for enzyme activity. Enzymatic decarboxylation is proposed to result from a combined action of the keto group of the enzyme-bound pyruvate protonated by an essential histidine and a protonated amino group of a lysine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
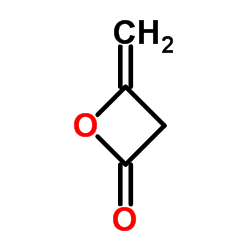 |
Diketene
CAS:674-82-8 |
C4H4O2 |
|
Chemical reactivity and biological activity of diketene.
2008-10-01 [Chem. Res. Toxicol. 21(10) , 1964-9, (2008)] |
|
Novel approach to 1,5-benzodiazepine-2-ones containing pepto...
2010-07-12 [J. Comb. Chem. 12(4) , 497-502, (2010)] |
|
One-pot multicomponent synthesis of 1-aryl-5-methyl-N-R2-1H-...
2009-01-01 [J. Comb. Chem. 11(3) , 481-5, (2009)] |
|
Interactions between earthworm hemolysins and sheep red bloo...
1989-08-07 [Biochim. Biophys. Acta 983(2) , 193-8, (1989)] |
|
New one-pot four-component synthesis of disubstituted pyrido...
2009-01-01 [J. Comb. Chem. 11(3) , 375-7, (2009)] |