Study of interaction between agonists and asn293 in helix VI of human beta(2)-adrenergic receptor.
H M Zuurmond, J Hessling, K Blüml, M Lohse, A P Ijzerman
Index: Mol. Pharmacol. 56 , 909, (1999)
Full Text: HTML
Abstract
Previously, we demonstrated the involvement of Asn293 in helix VI of the human beta(2)-adrenergic receptor in stereoselective agonist recognition and activation. In the present study, we have further explored the role of this residue by synthesizing derivatives of isoproterenol and clenbuterol, two beta-adrenergic receptor agonists. We analyzed their efficacy and affinity on the wild-type and a mutant receptor (Asn293Leu). Each compound had similar efficacy (tau values) on both the wild-type and mutant receptor, although tau values varied considerably among the eight compounds studied. It appeared that one derivative of isoproterenol, but not of clenbuterol, showed a gain in affinity from the wild type to the mutant receptor. This derivative had a methyl substituent instead of the usual beta-OH group in the aliphatic side chain of isoproterenol, compatible with the more lipophilic nature of the leucine side chain. Such a "gain of function" approach through a combination of synthetic chemistry with molecular biology, may be useful to enhance our insight into the precise atomic events that govern ligand-receptor interactions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
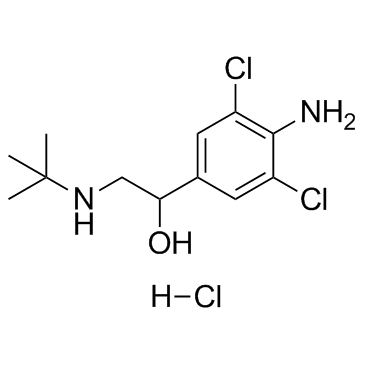 |
Clenbuterol hydrochloride
CAS:21898-19-1 |
C12H19Cl3N2O |
|
Mutagenicity and DNA-damaging potential of clenbuterol and i...
2015-03-01 [Food Chem. Toxicol. 77 , 82-92, (2015)] |
|
Detection of clenbuterol hydrochloride residuals in pork liv...
2015-01-01 [PLoS ONE 10(3) , e0122005, (2015)] |
|
The beta-adrenoceptor agonist clenbuterol is a potent inhibi...
1999-09-01 [Inflamm. Res. 48 , 497-502, (1999)] |
|
Neuroprotection mediated via neurotrophic factors and induct...
[Brain Res. Brain Res. Rev. 30 , 176-188, (1999)] |
|
Clenbuterol residues in pig muscle after repeat administrati...
2010-11-01 [Meat Science 86 , 733-7, (2010)] |