Quantitation of cysteine residues alkylated with 3-bromopropylamine by amino acid analysis.
J E Hale, D E Beidler, R A Jue
Index: Anal. Biochem. 216(1) , 61-6, (1994)
Full Text: HTML
Abstract
A new versatile reagent, 3-bromopropylamine, for the quantitative analysis of cysteine residues in proteins and peptides is reported. When added to amino acid standards, the 3-bromopropylamine derivative of cysteine, S-3-aminopropylcysteine, elutes in a unique position on four different amino acid analysis systems without modification to their standard gradients. Optimized conditions for the complete alkylation of cysteines in proteins with 3-bromopropylamine are described. The S-3-aminopropylcysteine is stable to standard acid hydrolysis conditions used for amino acid analysis. Cysteine values are within 10% of the predicted value in the amino acid analysis of acid hydrolysates of known proteins based on quantitation with S-3-aminopropylcysteine. No evidence of alkylation of other amino acids by 3-bromopropylamine is apparent from the amino acid analysis of proteins alkylated under the optimal conditions. These results expand the application of 3-bromopropylamine to include quantitation of cysteine by amino acid analysis as well as the previously reported identification of cysteines by protein sequencing.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
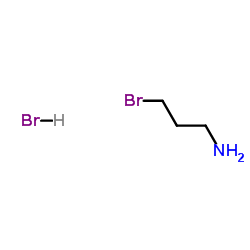 |
3-Bromopropylamine hydrobromide
CAS:5003-71-4 |
C3H9Br2N |
|
Direct Pore Binding as a Mechanism for Isoflurane Inhibition...
2015-01-01 [Sci. Rep. 5 , 13833, (2015)] |
|
Inhibition of tissue-bound semicarbazide-sensitive amine oxi...
2001-01-01 [Arch. Biochem. Biophys. 385(1) , 154-61, (2001)] |
|
Modification of cysteine.
2001-05-01 [Curr. Protoc. Protein Sci. Chapter 15 , Unit15.1, (2001)] |
|
Rational design of ligands targeting triplet repeating trans...
2009-07-22 [J. Am. Chem. Soc. 131(28) , 9767-79, (2009)] |
|
Inhibition of copper amine oxidase by haloamines: a killer p...
1997-03-04 [Biochemistry 36(9) , 2595-602, (1997)] |