| Structure | Name/CAS No. | Articles |
|---|---|---|
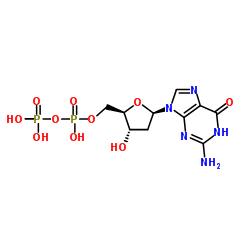 |
Deoxyguanosine diphosphate
CAS:102783-74-4 |
|
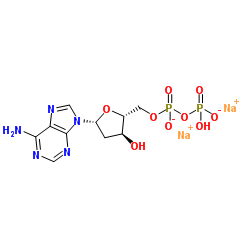 |
2'-Deoxyadenosine-5'-diphosphate disodium salt
CAS:72003-83-9 |
|
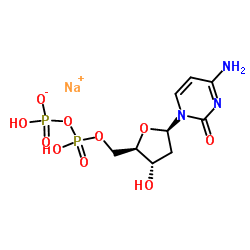 |
2'-Deoxycytidine-5'-diphosphate trisodium salt
CAS:151151-32-5 |