Biochemical characterization of Helicobacter pylori α-1,4 fucosyltransferase: metal ion requirement, donor substrate specificity and organic solvent stability.
Said Rabbani, Francesco Corona, Beat Ernst
Index: Biometals 22(6) , 1011-7, (2009)
Full Text: HTML
Abstract
The effect of metal ions on the activity, the donor substrate specificity, and the stability in organic solvents of Helicobacter pylori α-1,4 fucosyltransferase were studied. The recombinant enzyme was expressed as soluble form in E. coli strain AD494 and purified in a one step affinity chromatography. Its activity was highest in cacodylate buffer at pH 6.5 in the presence of 20 mM Mn2+ ions at 37°C. Mn2+ ions could be substituted by other metal ions. In all cases, Mn2+ ions proofed to be the most effective (Mn2+ > Co2+ > Ca2+ > Mg2+ > Cu2+ > Ni2+ > EDTA). The enzyme shows substrate specificity for Type I disaccharide (1) with a KM of 114 μM. In addition, the H. pylori α-1,4 fucosyltransferase efficiently transfers GDP-activated L-fucose derivatives to Galβ1-3GlcNAc-OR (1). Interestingly, the presence of organic solvents such as DMSO and methanol up to 20% in the reaction medium does not affect significantly the enzyme activity. However, at the same concentration of dioxane, activity is totally abolished.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
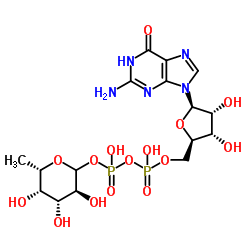 |
GDP-L-fucose disodium salt
CAS:15839-70-0 |
C16H23N5Na2O15P2 |
|
Low glucose depletes glycan precursors, reduces site occupan...
2015-07-01 [Biotechnol. J. 10 , 1051-66, (2015)] |
|
Chemo-enzymatic supported synthesis of the 3-sulfated Lewis ...
2008-04-07 [Carbohydr. Res. 343(5) , 970-6, (2008)] |
|
Structures of NodZ α1,6-fucosyltransferase in complex with G...
2012-02-01 [Acta Crystallogr. D Biol. Crystallogr. 68 , 160-168, (2012)] |
|
Biochemical characteristics and function of a fucosyltransfe...
2009-10-01 [J. Microbiol. Biotechnol. 19(10) , 1092-7, (2009)] |
|
Two pathways for importing GDP-fucose into the endoplasmic r...
2010-02-05 [J. Biol. Chem. 285(6) , 4122-9, (2010)] |