| Structure | Name/CAS No. | Articles |
|---|---|---|
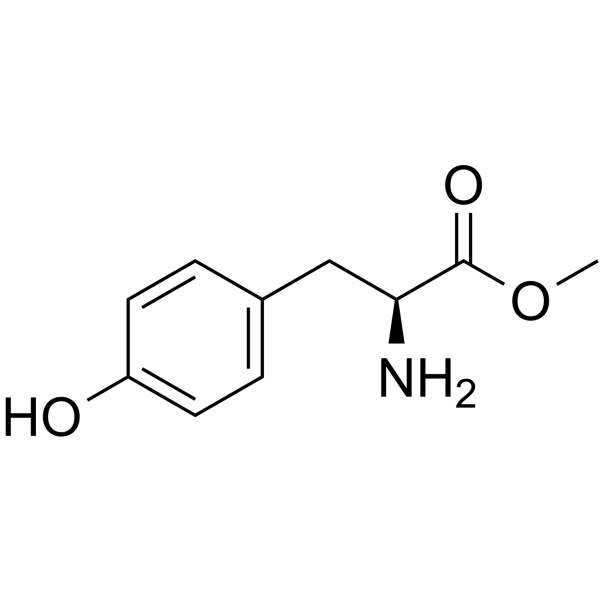 |
H-Tyr-OMe
CAS:1080-06-4 |
|
 |
m-phthaloyl chloride
CAS:99-63-8 |
|
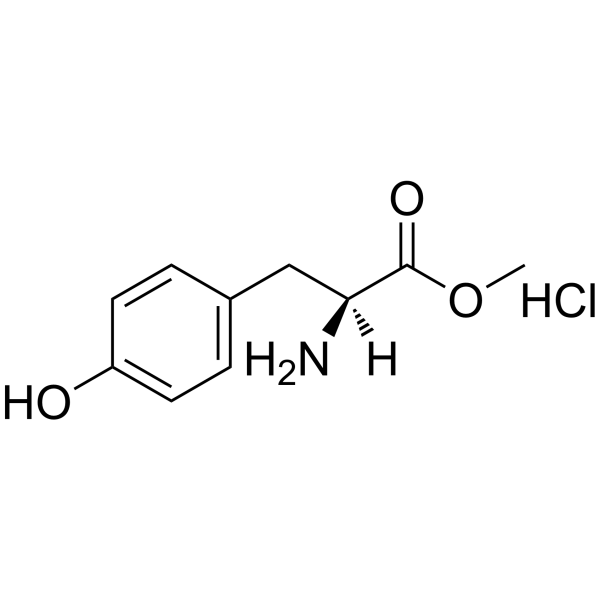 |
H-Tyr-OMe.HCl
CAS:3417-91-2 |