| Structure | Name/CAS No. | Articles |
|---|---|---|
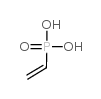 |
Vinylphosphonic acid
CAS:1746-03-8 |
|
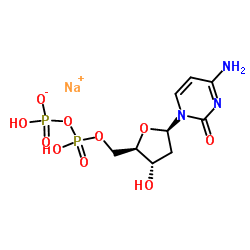 |
2'-Deoxycytidine-5'-diphosphate trisodium salt
CAS:151151-32-5 |