| Structure | Name/CAS No. | Articles |
|---|---|---|
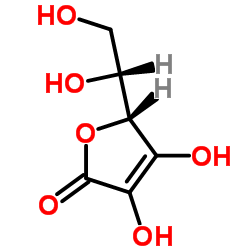 |
Erythorbic acid
CAS:89-65-6 |
|
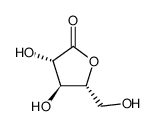 |
D-ARABINO-1,4-LACTONE
CAS:2782-09-4 |