Theoretical and experimental vibrational study of the phenyl urea herbicide 1-(4-bromo-3-chlorophenyl)-3-methoxy-3-methylurea.
J Clemy Monicka, C James
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 94 , 30-5, (2012)
Full Text: HTML
Abstract
FT-Raman and IR spectra of the herbicidal compound chlorbromuron have been recorded and analyzed. The detailed interpretation of the vibrational spectra has been carried out with the aid of normal coordinate analysis (NCA) following the scaled quantum mechanical force field methodology. The various intramolecular interactions which are responsible for the stabilization of the molecule were revealed by natural bond orbital analysis. The Mulliken population analysis on atomic charges and the HOMO-LUMO energy were also calculated. The presence of strong NH⋯O intermolecular hydrogen bonding was clearly exposed from the IR spectrum by the red shifting of NH stretching wavenumber.Copyright © 2012 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
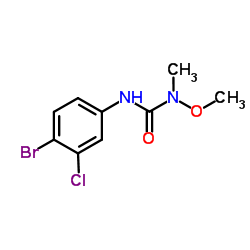 |
3-(4-Bromo-3-chlorophenyl)-1-methoxy-1-methylurea
CAS:13360-45-7 |
C9H10BrClN2O2 |
|
[On the determination of chlorpropham, metobromuron and chlo...
1982-05-01 [Pharmazie 37(5) , 370-4, (1982)] |
|
[Thin-layer chromatographic testing of drugs of the East Ger...
1981-04-01 [Pharmazie 36(4) , 304, (1981)] |
|
[Gas chromatographic determination of chlorpropham, metobrom...
1981-05-01 [Pharmazie 36(5) , 386-7, (1981)] |
|
Toxicity of the herbicides Flubalex, Fusilade S and Maloran ...
1996-01-01 [Acta Vet. Hung. 44(3) , 363-76, (1996)] |
|
[Hygienic standardization of the herbicide Maloran in the ai...
1981-08-01 [Gig. Sanit. (8) , 76-8, (1981)] |