Matrix isolation and computational study of the photochemistry of p-azidoaniline.
Elena A Pritchina, Nina P Gritsan, Thomas Bally
Index: Phys. Chem. Chem. Phys. 8(6) , 719-27, (2006)
Full Text: HTML
Abstract
The photochemistry of p-azidoaniline was studied in argon matrices in the absence and presence of oxygen. With the help of quantum chemical calculations we were able to characterize the triplet p-aminophenylnitrene as well as the cis- and trans-p-aminophenylnitroso oxides. It was found that the latter two isomers can be interconverted by selective irradiation and that they are ultimately converted into p-nitroaniline. Although restricted wavefunctions of the nitroso oxides are unstable, CASSCF calculations turned up no evidence for the claimed diradical character of these compounds. Also we found no evidence for dioxaziridines as intermediates of the conversion of the nitroso oxides to p-nitroaniline.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
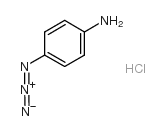 |
4-Azidoaniline hydrochloride
CAS:91159-79-4 |
C6H7ClN4 |
|
[Study on immobilization of heparin on surface of Ti-O films...
2011-02-01 [Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 28(1) , 86-9, (2011)] |
|
Peptidotriazoles on solid phase: [1,2,3]-triazoles by regios...
2002-05-03 [J. Org. Chem. 67(9) , 3057-64, (2002)] |
|
Preparation of a protein micro-array using a photo-reactive ...
2003-08-01 [Biomaterials 24(18) , 3021-6, (2003)] |
|
The use of hyaluronan and its sulphated derivative patterned...
2003-03-01 [Biomaterials 24(6) , 915-26, (2003)] |
|
Enhancing neurite outgrowth from primary neurones and neural...
2009-04-01 [J. Biomed. Mater. Res. A 89(1) , 24-35, (2009)] |