Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides.
Christian W Tornøe, Caspar Christensen, Morten Meldal
Index: J. Org. Chem. 67(9) , 3057-64, (2002)
Full Text: HTML
Abstract
The cycloaddition of azides to alkynes is one of the most important synthetic routes to 1H-[1,2,3]-triazoles. Here a novel regiospecific copper(I)-catalyzed 1,3-dipolar cycloaddition of terminal alkynes to azides on solid-phase is reported. Primary, secondary, and tertiary alkyl azides, aryl azides, and an azido sugar were used successfully in the copper(I)-catalyzed cycloaddition producing diversely 1,4-substituted [1,2,3]-triazoles in peptide backbones or side chains. The reaction conditions were fully compatible with solid-phase peptide synthesis on polar supports. The copper(I) catalysis is mild and efficient (>95% conversion and purity in most cases) and furthermore, the X-ray structure of 2-azido-2-methylpropanoic acid has been solved, to yield structural information on the 1,3-dipoles entering the reaction. Novel Fmoc-protected amino azides derived from Fmoc-amino alcohols were prepared by the Mitsunobu reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
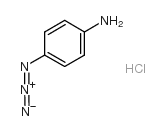 |
4-Azidoaniline hydrochloride
CAS:91159-79-4 |
C6H7ClN4 |
|
[Study on immobilization of heparin on surface of Ti-O films...
2011-02-01 [Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 28(1) , 86-9, (2011)] |
|
Preparation of a protein micro-array using a photo-reactive ...
2003-08-01 [Biomaterials 24(18) , 3021-6, (2003)] |
|
The use of hyaluronan and its sulphated derivative patterned...
2003-03-01 [Biomaterials 24(6) , 915-26, (2003)] |
|
Enhancing neurite outgrowth from primary neurones and neural...
2009-04-01 [J. Biomed. Mater. Res. A 89(1) , 24-35, (2009)] |
|
Characterization and photoaffinity labeling of a calcitonin ...
1991-09-03 [Biochemistry 30(35) , 8605-11, (1991)] |