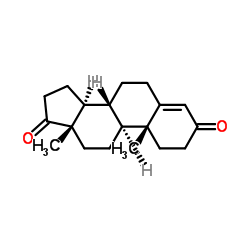
|
~% |
|
~21% |
|
~% |
|
~% |
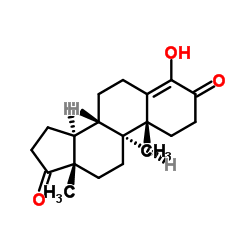

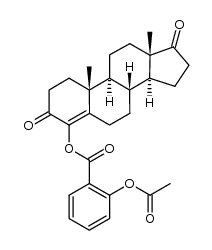
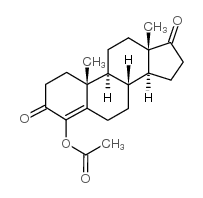
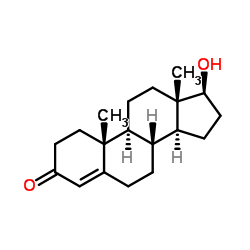
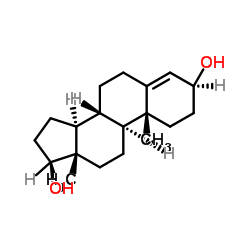
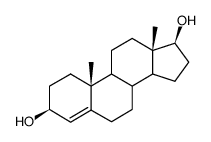
![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,2,5,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one Structure](https://image.chemsrc.com/caspic/321/14935-81-0.png)