Critical role of the H6-H7 loop in the conformational adaptation of all-trans retinoic acid and synthetic retinoids within the ligand-binding site of RARalpha.
S Mailfait, E Thoreau, D Belaiche, P Formstecher And B Sablonniè
Index: J. Mol. Endocrinol. 24(3) , 353-64, (2000)
Full Text: HTML
Abstract
The pleiotropic effects of the natural and synthetic retinoids are mediated by the activation of the two subfamilies of nuclear receptors, the retinoic acid receptors (RARs) and the retinoic X receptors (RXRs). At the molecular level, these events begin with the specific ligand recognition by a nuclear receptor subtype. The adaptation of ligands to the receptor binding site leads to an optimal number of interactions for binding and selectivity which justifies elucidation of the structural requirements of the ligand binding pocket. To explore the contribution of H6-H7 loop folding in the ligand-induced conformational changes explained by the mouse-trap model, four RARalpha mutants were constructed. Ligand binding and transactivation studies revealed that three residues from the H6-H7 loop (Gly(301), Phe(302) and Gly(303)) are critical for the conformational adaptation of both synthetic agonists and antagonists. Model building and analysis of both RARalpha-ATRA and RARalpha-CD367 complexes demonstrate that accommodation of CD367 results in a less tight contact of the saturated ring of this ligand with the amino acid side chains of the receptor ligand-binding pocket compared with that of ATRA. According to the flexibility of the agonists tested (ATRA>TTNPB=Am580> CD367), we observed a decrease in binding that was dependent on ligand structure rigidity. In contrast, the binding and transactivating activities of the L266A mutant confirmed the structural constraints imposed by synthetic ligands on binding affinity for the receptor and revealed that subtle local rearrangements induced by specific conformational adaptation changes result in different binding affinities. Our results illustrate the dynamic nature of the interaction between RARalpha and its ligands and demonstrate the critical role of the H6-H7 loop in the binding of both synthetic retinoid agonists and antagonists.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
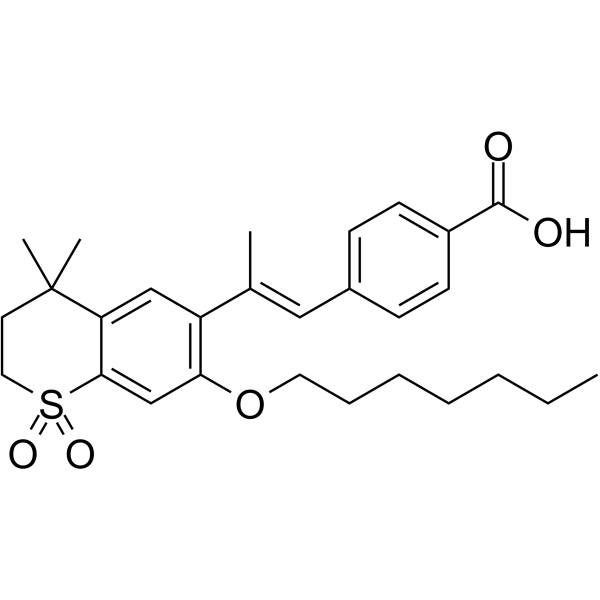 |
Ro 41-5253
CAS:144092-31-9 |
C28H36O5S |
|
Effect of all-trans-retinoic acid on enterovirus 71 infectio...
2014-05-01 [Br. J. Nutr. 111(9) , 1586-93, (2014)] |
|
Retinoic acid inhibits in vivo interleukin-2 gene expression...
2009-04-01 [Immunology 126(4) , 514-22, (2009)] |
|
Overexpression of mucin genes induced by interleukin-1 beta,...
2002-06-01 [Exp. Lung Res. 28(4) , 315-32, (2002)] |
|
Retinoic acid signaling is necessary for the development of ...
1999-09-01 [Dev. Biol. 213(1) , 180-93, (1999)] |
|
Retinoids and human breast cancer: in vivo effects of an ant...
2005-02-28 [Cancer Lett. 219(1) , 27-31, (2005)] |