Synthesis of pyrazoles via electrophilic cyclization.
Metin Zora, Arif Kivrak, Ceyda Yazici
Index: J. Org. Chem. 76(16) , 6726-42, (2011)
Full Text: HTML
Abstract
Electrophilic cyclizations of α,β-alkynic hydrazones by molecular iodine were investigated for the synthesis of 4-iodopyrazoles. α,β-Alkynic hydrazones were readily prepared by the reactions of hydrazines with propargyl aldehydes and ketones. When treated with molecular iodine in the presence of sodium bicarbonate, α,β-alkynic hydrazones underwent electrophilic cyclization to afford 4-iodopyrazoles in good to high yields. Iodocyclization was general for a wide range of α,β-alkynic hydrazones and tolerated the presence of aliphatic, aromatic, heteroaromatic, and ferrocenyl moieties with electron-withdrawing and electron-donating substituents.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
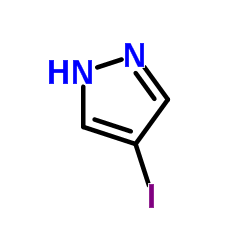 |
4-Iodopyrazole
CAS:3469-69-0 |
C3H3IN2 |
|
Structures of three human beta alcohol dehydrogenase variant...
1994-06-10 [J. Mol. Biol. 239(3) , 415-29, (1994)] |
|
The effects of caffeic acid phenethyl ester in acute methano...
2013-10-01 [Cutan. Ocul. Toxicol. 32(4) , 263-7, (2013)] |
|
Indium-mediated synthesis of heterobiaryls.
2007-04-27 [J. Org. Chem. 72 , 3589, (2007)] |
|
Some iodinated pyrazole derivatives.
1966-01-01 [J. Chem. Soc. Perkin Trans. I 13 , 1179-84, (1966)] |
|
Pyrazole binding in crystalline binary and ternary complexes...
1982-09-28 [Biochemistry 21(20) , 4858-66, (1982)] |