Organic Letters
2012-01-20
Organocatalytic Michael-Knoevenagel-hetero-Diels-Alder reactions: an efficient asymmetric one-pot strategy to isochromene pyrimidinedione derivatives.
Bor-Cherng Hong, Nitin S Dange, Chun-Feng Ding, Ju-Hsiou Liao
Index: Org. Lett. 14(2) , 448-51, (2012)
Full Text: HTML
Abstract
Synthesis of isochromene pyrimidinedione derivatives having five stereocenters has been achieved by a one-pot Michael-Knoevenagel condensation-inverse-electron-demand hetero-Diels-Alder reaction of α, β-unsaturated aldehydes, olefinic nitroalkanes, and 1,3-dimethylbarbituric acid via a one-pot strategy with excellent diastereo- and enantioselectivities (up to 99% ee). The structures and absolute configurations of the products were confirmed by X-ray analysis.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
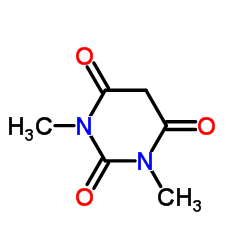 |
1,3-dimethylbarbituric acid
CAS:769-42-6 |
C6H8N2O3 |
Related Articles:
More...
|
Self-assembled Pd6 open cage with triimidazole walls and the...
2012-09-24 [Chemistry 18(39) , 12322-9, (2012)] |
|
Multicomponent self-sorting of a Pd7 molecular boat and its ...
2013-05-14 [Chem. Commun. (Camb.) 49(39) , 4307-9, (2013)] |
|
Synthesis of 5-aryl-1,3-dimethyl-6-(alkyl- or aryl-amino) fu...
2011-02-01 [Mol. Divers. 15(1) , 227-31, (2011)] |
|
Sequential one-pot bimetallic Ir(III)/Pd(0) catalysed mono-/...
2006-12-28 [Chem. Commun. (Camb.) (48) , 5000-2, (2006)] |
|
Experimental and computational thermochemical study of barbi...
2011-04-14 [J. Phys. Chem. A 115(14) , 3167-73, (2011)] |