Understanding acoustic cavitation for sonolytic degradation of p-cresol as a model contaminant.
Rajesh Balachandran, Zach Patterson, Pierre Deymier, Shane A Snyder, Manish Keswani
Index: Chemosphere 147 , 52-9, (2016)
Full Text: HTML
Abstract
Many modern techniques exist for the degradation of organic pollutants in water. Numerous treatment processes which utilize the formation of hydroxyl radicals for oxidation of pollutants have been studied thoroughly. In this study, a three pronged approach has been used to characterize and understand the effect of two distinct acoustic frequencies (37 kHz and 1 MHz) on cavitation behavior. Correlation of this behavior with sonolysis of a target phenol pollutant is described. Hydroxyl radical capture, hydrophone, and microelectrode studies in this work show that megasonic frequencies are more effective for generation of hydroxyl radicals and stable cavitation events than ultrasonic frequencies. UV absorption and fluorescence measurements confirm that the combination of ultrasonic sonolysis with a Fenton reagent achieved complete degradation of p-cresol at 50 mg/L in about 30 min. Cost estimates have been made for different sonication processes and compared with traditional advanced oxidation processes.Copyright © 2015 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
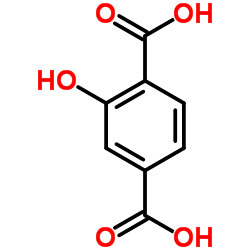 |
2-Hydroxyterephthalic acid
CAS:636-94-2 |
C8H6O5 |
|
Hydrothermal synthesis, phase structure, optical and photoca...
2015-11-01 [J. Colloid. Interface Sci. 457 , 360-9, (2015)] |
|
Involutin is an Fe3+ reductant secreted by the ectomycorrhiz...
2015-12-01 [Appl. Environ. Microbiol. 81 , 8427-33, (2015)] |
|
A rapid and sensitive method for hydroxyl radical detection ...
2014-07-07 [Analyst 139(13) , 3416-22, (2014)] |
|
A high-throughput assay for enzymatic polyester hydrolysis a...
2012-12-01 [Biotechnol. J. 7(12) , 1517-21, (2012)] |
|
Hydroxyterephthalate as a fluorescent probe for hydroxyl rad...
2000-03-01 [Photochem. Photobiol. 71(3) , 307-13, (2000)] |