Flexibility and sorption selectivity in rigid metal-organic frameworks: the impact of ether-functionalised linkers.
Sebastian Henke, Rochus Schmid, Jan-Dierk Grunwaldt, Roland A Fischer
Index: Chemistry 16 , 14296, (2010)
Full Text: HTML
Abstract
The functionalisation of well-known rigid metal-organic frameworks (namely, [Zn(4)O(bdc)(3)](n), MOF-5, IRMOF-1 and [Zn(2)(bdc)(2)(dabco)](n); bdc = 1,4-benzene dicarboxylate, dabco = diazabicyclo[2.2.2]octane) with additional alkyl ether groups of the type -O-(CH(2))(n)-O-CH(3) (n = 2-4) initiates unexpected structural flexibility, as well as high sorption selectivity towards CO(2) over N(2) and CH(4) in the porous materials. These novel materials respond to the presence/absence of guest molecules with structural transformations. We found that the chain length of the alkyl ether groups and the substitution pattern of the bdc-type linker have a major impact on structural flexibility and sorption selectivity. Remarkably, our results show that a high crystalline order of the activated material is not a prerequisite to achieve significant porosity and high sorption selectivity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
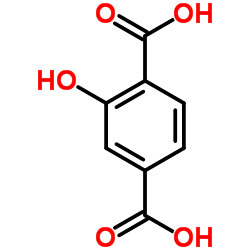 |
2-Hydroxyterephthalic acid
CAS:636-94-2 |
C8H6O5 |
|
Hydrothermal synthesis, phase structure, optical and photoca...
2015-11-01 [J. Colloid. Interface Sci. 457 , 360-9, (2015)] |
|
Involutin is an Fe3+ reductant secreted by the ectomycorrhiz...
2015-12-01 [Appl. Environ. Microbiol. 81 , 8427-33, (2015)] |
|
A rapid and sensitive method for hydroxyl radical detection ...
2014-07-07 [Analyst 139(13) , 3416-22, (2014)] |
|
A high-throughput assay for enzymatic polyester hydrolysis a...
2012-12-01 [Biotechnol. J. 7(12) , 1517-21, (2012)] |
|
Hydroxyterephthalate as a fluorescent probe for hydroxyl rad...
2000-03-01 [Photochem. Photobiol. 71(3) , 307-13, (2000)] |