Intramolecular hydrogen bonding (proton transfer) of 1-phenyl-1,3-butanedione.
Hideyo Matsuzawa, Takashi Nakagaki, Makio Iwahashi
Index: J. Oleo Sci. 56(12) , 653-8, (2007)
Full Text: HTML
Abstract
Through the (1)H and (13)C NMR measurements for the symmetrical beta-diketones such as 2,4-pentanedione and 1,3-diphenyl-1,3-propanedione and unsymmetrical one such as 1-phenyl-1,3-butanedione at various concentrations and temperatures, we confirmed that 1-phenyl-1,3-butanedione in CDCl(3) exists as monomers in its relatively low concentration. In addition, the 1-phenyl-1,3-butanedione in CDCl(3) exists not as a keto-form but as two kinds of cis-enol forms. The proton transfer between the two kinds of cis-enols for 1-phenyl-1,3-butanedione was discussed thermodynamically; it is concluded that the OH proton of enol of 1-phenyl-1,3-butanedione is considerably located near the oxygen atom attached to the carbon atom linking to a phenyl group.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
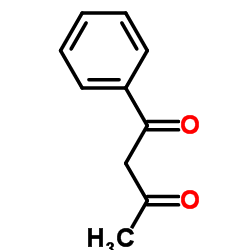 |
1-Phenylbutane-1,3-dione
CAS:93-91-4 |
C10H10O2 |
|
Synthesis, Characterization and Biological Studies of Metal(...
2015-01-01 [Molecules 20 , 9788-802, (2015)] |
|
Potential anticonvulsants IV: Condensation of isatin with be...
1982-09-01 [J. Pharm. Sci. 71(9) , 1052-4, (1982)] |
|
The sensitive determination of nucleic acids using fluoresce...
2005-09-01 [J. Fluoresc. 15(5) , 655-60, (2005)] |
|
Tautomeric and conformational properties of benzoylacetone, ...
2012-04-05 [J. Phys. Chem. A 116(13) , 3428-35, (2012)] |
|
On the electronic nature of low-barrier hydrogen bonds in en...
1998-10-27 [Proc. Natl. Acad. Sci. U. S. A. 95(22) , 12799-802, (1998)] |