Tautomeric and conformational properties of benzoylacetone, CH3-C(O)-CH2-C(O)-C6H5: gas-phase electron diffraction and quantum chemical study.
Natalya V Belova, Georgiy V Girichev, Heinz Oberhammer, Trang Nguen Hoang, Sergey A Shlykov
Index: J. Phys. Chem. A 116(13) , 3428-35, (2012)
Full Text: HTML
Abstract
Tautomeric and structural properties of benzoylacetone, CH(3)-C(O)-CH(2)-C(O)-C(6)H(5), have been studied by gas-phase electron diffraction (GED) and quantum chemical calculations (B3LYP and MP2 approximation with different basis sets up to aug-cc-pVTZ). Analysis of GED intensities resulted in the presence of 100% enol tautomer at 331(5) K. The existence of two possible enol conformers in about equal amounts is confirmed by both GED and quantum chemical results. In both conformers the enol ring possesses C(s) symmetry with a strongly asymmetric hydrogen bond. The experimental geometric parameters are reproduced very closely by the B3LYP/cc-pVTZ method.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
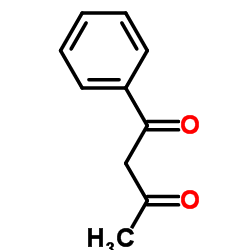 |
1-Phenylbutane-1,3-dione
CAS:93-91-4 |
C10H10O2 |
|
Synthesis, Characterization and Biological Studies of Metal(...
2015-01-01 [Molecules 20 , 9788-802, (2015)] |
|
Potential anticonvulsants IV: Condensation of isatin with be...
1982-09-01 [J. Pharm. Sci. 71(9) , 1052-4, (1982)] |
|
The sensitive determination of nucleic acids using fluoresce...
2005-09-01 [J. Fluoresc. 15(5) , 655-60, (2005)] |
|
On the electronic nature of low-barrier hydrogen bonds in en...
1998-10-27 [Proc. Natl. Acad. Sci. U. S. A. 95(22) , 12799-802, (1998)] |
|
Vibrational assignment and structure of dibenzoylmethane. A ...
2007-02-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(2) , 394-404, (2007)] |