Organic Letters
2006-03-30
Catalytic asymmetric addition of dimethylzinc to alpha-ketoesters, using mandelamides as ligands.
Gonzalo Blay, Isabel Fernandez, Alícia Marco-Aleixandre, José R Pedro
Index: Org. Lett. 8(7) , 1287-90, (2006)
Full Text: HTML
Abstract
[reaction: see text] A strategy based on the control of the electron-donating capabilities of the coordinating groups of the ligand has been applied in the catalytic asymmetric addition of organometallic reagents to ketoesters. Mandelamides having deprotonated alcohol and carboxyamido groups catalyzed the addition of dimethylzinc to alpha-ketoesters with good yields and ee (up to 90%).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
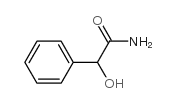 |
(+/-)-Mandelamide
CAS:4410-31-5 |
C8H9NO2 |
Related Articles:
More...
|
The mandelamide keto-enol system in aqueous solution. Genera...
2003-01-08 [J. Am. Chem. Soc. 125(1) , 187-94, (2003)] |
|
[Molecular biology aspects of the antimicrobial effect of sy...
1982-08-01 [Z. Arztl. Fortbild. (Jena.) 76(15) , 662-6, (1982)] |
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propar...
2006-05-01 [Pest Manag. Sci. 62(5) , 446-51, (2006)] |
|
Synthesis and fungicidal activity of N-2-(3-methoxy-4-propar...
2007-01-01 [Pest Manag. Sci. 63(1) , 57-62, (2007)] |
|
Identification of amino acid residues responsible for the en...
2009-09-01 [Appl. Environ. Microbiol. 75(17) , 5592-9, (2009)] |