Role of human UDP-glucuronosyltransferases in the biotransformation of the triazoloacridinone and imidazoacridinone antitumor agents C-1305 and C-1311: highly selective substrates for UGT1A10.
Barbara Fedejko-Kap, Stacie M Bratton, Moshe Finel, Anna Radominska-Pandya, Zofia Mazerska
Index: Drug Metab. Dispos. 40(9) , 1736-43, (2012)
Full Text: HTML
Abstract
5-Diethylaminoethylamino-8-hydroxyimidazoacridinone, C-1311 (NSC-645809), is an antitumor agent shown to be effective against breast cancer in phase II clinical trials. A similar compound, 5-dimethylaminopropylamino-8-hydroxytriazoloacridinone, C-1305, shows high activity against experimental tumors and is expected to have even more beneficial pharmacological properties than C-1311. Previously published studies showed that these compounds are not substrates for cytochrome P450s; however, they do contain functional groups that are common targets for glucuronidation. Therefore, the aim of this work was to identify the human UDP-glucuronosyltransferases (UGTs) able to glucuronidate these two compounds. High-performance liquid chromatography analysis was used to examine the activities of human recombinant UGT1A and UGT2B isoforms and microsomes from human liver [human liver microsomes (HLM)], whole human intestinal mucosa [human intestinal microsomes (HIM)], and seven isolated segments of human gastrointestinal tract. Recombinant extrahepatic UGT1A10 glucuronidated 8-hydroxyl groups with the highest catalytic efficiency compared with other recombinant UGTs, V(max)/K(m) = 27.2 and 8.8 μl · min⁻¹ · mg protein⁻¹, for C-1305 and C-1311, respectively. In human hepatic and intestinal microsomes (HLM and HIM, respectively), high variability in UGT activities was observed among donors and for different regions of intestinal tract. However, both compounds underwent UGT-mediated metabolism to 8-O-glucuronides by microsomes from both sources with comparable efficiency; V(max)/K(m) values were from 4.0 to 5.5 μl · min⁻¹ · mg protein⁻¹. In summary, these studies suggest that imid azoacridinone and triazoloacridinone drugs are glucuronidated in human liver and intestine in vivo and may form the basis for future translational studies of the potential role of UGTs in resistance to these drugs.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
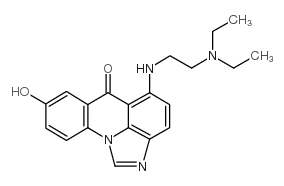 |
C-1311
CAS:138154-39-9 |
C20H22N4O2 |
|
DNA-damaging imidazoacridinone C-1311 induces autophagy foll...
2013-09-01 [J. Pharmacol. Exp. Ther. 346(3) , 393-405, (2013)] |
|
Metabolic transformation of antitumor acridinone C-1305 but ...
2013-02-01 [Drug Metab. Dispos. 41(2) , 414-21, (2013)] |
|
Development and validation of an LC-UV method for the quanti...
2005-09-01 [J. Pharm. Biomed. Anal. 39(1-2) , 46-53, (2005)] |
|
Thermoresponsive polymeric gel as a medium for examining int...
2008-10-01 [Anal. Bioanal. Chem 392(3) , 463-9, (2008)] |
|
Flavin monooxygenases, FMO1 and FMO3, not cytochrome P450 is...
2011-12-01 [Xenobiotica 41(12) , 1044-55, (2011)] |