Thermoresponsive polymeric gel as a medium for examining interactions between dsDNA and an anticancer drug.
Agata Kowalczyk, Anna M Nowicka, Marcin Karbarz, Zbigniew Stojek
Index: Anal. Bioanal. Chem 392(3) , 463-9, (2008)
Full Text: HTML
Abstract
A piece of dry N-isopropylacrylamide polymer was soaked in phosphate buffer to obtain a hydrogel which was then employed in the examination of interactions between an anticancer drug C-1311 (5-diethylaminoethyl-amino-8-hydroxyimidazoacridinone) and dsDNA. dsDNA was introduced into the polymer at the polymerization stage. The drug was added to the buffer. Using the volume phase transition of the gel at 40 degrees C, the unbound drug could be determined in the solution released during the transition, which made the calculations more reliable. The interaction parameters were calculated using the McGhee and von Hippel model. It appeared that in the gel medium, the interaction between the drug and dsDNA is spatially limited, since the number of binding units of the polymer chain occupied by one drug molecule was found to be one, while it was two in the regular buffer solution.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
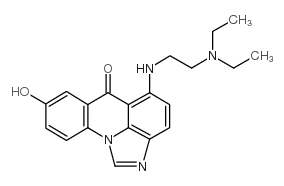 |
C-1311
CAS:138154-39-9 |
C20H22N4O2 |
|
DNA-damaging imidazoacridinone C-1311 induces autophagy foll...
2013-09-01 [J. Pharmacol. Exp. Ther. 346(3) , 393-405, (2013)] |
|
Metabolic transformation of antitumor acridinone C-1305 but ...
2013-02-01 [Drug Metab. Dispos. 41(2) , 414-21, (2013)] |
|
Development and validation of an LC-UV method for the quanti...
2005-09-01 [J. Pharm. Biomed. Anal. 39(1-2) , 46-53, (2005)] |
|
Flavin monooxygenases, FMO1 and FMO3, not cytochrome P450 is...
2011-12-01 [Xenobiotica 41(12) , 1044-55, (2011)] |
|
Anticancer imidazoacridinone C-1311 inhibits hypoxia-inducib...
2011-10-01 [Cancer Biol. Ther. 12(7) , 586-97, (2011)] |