Synthesis of novel triplet drugs with 1,3,5-trioxazatriquinane skeletons and their pharmacologies. Part 2: Synthesis of novel triplet drugs with the epoxymethano structure (capped homotriplet).
Hiroshi Nagase, Koji Koyano, Naohisa Wada, Shigeto Hirayama, Akio Watanabe, Toru Nemoto, Mayumi Nakajima, Kaoru Nakao, Hidenori Mochizuki, Hideaki Fujii
Index: Bioorg. Med. Chem. Lett. 21(20) , 6198-202, (2011)
Full Text: HTML
Abstract
An improved synthetic method for triplet drugs with the 1,3,5-trioxazatriquinane skeleton was developed that used p-toluenesulfonylmethyl isocyanide (TosMIC) instead of 1,3-dithiane. Using the improved method, we synthesized compounds with two identical pharmacophore units and an epoxymethano group, that is, capped homotriplets. Among the synthesized capped homotriplets, KNT-123 showed high selectivity for the μ receptor over the κ receptor, and the μ selectivity was the highest among the reported μ selective nonpeptide ligands. KNT-123 administered subcutaneously induced a dose-dependent analgesic effect in the acetic acid writhing assay, and its potency was 11-fold more potent than that of morphine. KNT-123 may serve as a useful tool for the study of the pharmacological actions mediated specifically via the μ receptor.Copyright © 2011 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
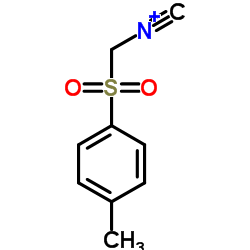 |
Tosylmethyl isocyanide
CAS:36635-61-7 |
C9H9NO2S |
|
Microwave-assisted synthesis of imidazoles: reaction of p-to...
2005-08-15 [Bioorg. Med. Chem. Lett. 15(16) , 3717-9, (2005)] |
|
Alkyl and aromatic isocyanide binding to haem complexes.
1989-09-15 [Biochem. J. 262(3) , 959-63, (1989)] |
|
[Med. Chem. Res. 3 , 192, (1993)] |
|
[Il Farmaco 48 , 209, (1993)] |
|
[Tetrahedron Lett. 48 , 5977, (2006)] |