Microwave-assisted synthesis of imidazoles: reaction of p-toluenesulfonylmethyl isocyanide and polymer-bound imines.
Swapan K Samanta, Irene Kylänlahti, Jari Yli-Kauhaluoma
Index: Bioorg. Med. Chem. Lett. 15(16) , 3717-9, (2005)
Full Text: HTML
Abstract
A convenient method for the synthesis of 1,5-disubstituted imidazoles has been developed on a polymeric support using base-promoted 1,3-dipolar cycloaddition reaction of p-toluenesulfonylmethyl isocyanide (TOSMIC) with immobilized imines under microwave irradiation. The immobilized imines were synthesized by the reaction of various primary benzyl amines with 4-formyl-3-methoxyphenoxymethyl polystyrene in the presence of trimethyl orthoformate at room temperature. Cleavage from the polymeric support using trifluoroacetic acid gave the desired 1,5-disubstituted imidazoles with excellent yield and high purity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
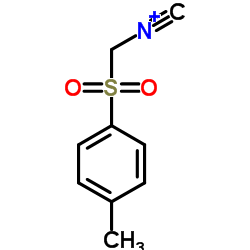 |
Tosylmethyl isocyanide
CAS:36635-61-7 |
C9H9NO2S |
|
Synthesis of novel triplet drugs with 1,3,5-trioxazatriquina...
2011-10-15 [Bioorg. Med. Chem. Lett. 21(20) , 6198-202, (2011)] |
|
Alkyl and aromatic isocyanide binding to haem complexes.
1989-09-15 [Biochem. J. 262(3) , 959-63, (1989)] |
|
[Med. Chem. Res. 3 , 192, (1993)] |
|
[Il Farmaco 48 , 209, (1993)] |
|
[Tetrahedron Lett. 48 , 5977, (2006)] |