Physiological concentrations of divalent magnesium ion activate the serine/threonine specific protein kinase ERK2.
William F Waas, Kevin N Dalby
Index: Biochemistry 42(10) , 2960-70, (2003)
Full Text: HTML
Abstract
Extracellular regulated protein kinase 2 (ERK2) is a eukaryotic protein kinase whose activity is regulated by phorbol esters, serum, and growth factors, and displays enhanced activity in several human tumors. Despite its important biological function, its mechanism of catalysis and mode of regulation are poorly understood. Recently, we showed that in the presence of 10 mM magnesium chloride, ERK2 phosphorylates the transcription factor Ets-1 through a random-ordered ternary-complex mechanism [Waas, W. F., and Dalby, K. N. (2002) J. Biol. Chem. 277, 12532]. Now we provide kinetic evidence that ERK2 must bind two divalent magnesium ions to facilitate catalysis at a physiologically relevant rate, because a second magnesium ion promotes both MgATP2- binding and phosphoryl transfer. The velocity dependence on magnesium at saturating concentrations of the protein substrate, Ets138, over a range of ATP4- and Mg2+ ion concentrations, supports the notion that magnesium is an essential activator of ERK2. At high (> or = 1 mM) concentrations of ATP4-, the velocity dependence on total Mg2+ is sigmoidal, but plateaus at high concentrations of free Mg2+, where the enzyme is fully activated. At concentrations of Mg2+ of < or = 4 mM, the velocity dependence on ATP4- displays a peak when the concentration of ATP4- approaches that of total Mg2+ and tends to zero at high concentrations of ATP4-, where the enzyme is predominantly unactivated. The observed velocity dependencies are consistent with the notion that ERK2*Etsdelta138 complexes and ATP4- compete for the same pool of Mg2+ ions in solution. No binding of ATP4- (0-2.5 mM) by ERK2 (65 microM) can be detected using isothermal titration calorimetry at 27 degrees C, pH 8.0, and an ionic strength of 0.15 M (KCl), suggesting that the complex, MgATP2-, is the true substrate for ERK2. In contrast, 5-iodotubericidin binds ERK2 tightly (K(d) = 1 microM) and displays a competitive inhibition pattern toward MgATP2- and a mixed pattern toward free Mg2+, suggesting that the binding of Mg2+ before MgATP2- is not compulsory.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
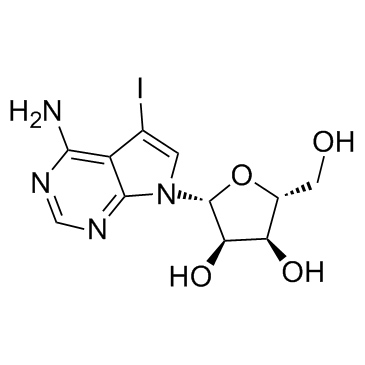 |
5-Iodotubercidin
CAS:24386-93-4 |
C11H13IN4O4 |
|
Chaperoning of the A1-adenosine receptor by endogenous adeno...
2015-01-01 [Mol. Pharmacol. 87(1) , 39-51, (2015)] |
|
Endothelial and Neuronal Nitric Oxide Activate Distinct Path...
2015-01-01 [PLoS ONE 10 , e0129224, (2015)] |
|
An adenosine kinase inhibitor, ABT-702, inhibits spinal noci...
2015-10-01 [Neuropharmacology 97 , 160-70, (2015)] |
|
Inosine strongly enhances proliferation of human C32 melanom...
2015-01-01 [Basic Clin Pharmacol Toxicol. 116(1) , 25-36, (2014)] |
|
The NLRP3 inflammasome is activated by nanoparticles through...
2015-01-01 [Cell Death Dis. 6 , e1629, (2015)] |