The reaction of cytochromes c and c2 with the Rhodospirillum rubrum reaction center involves the heme crevice domain.
J Hall, M Ayres, X H Zha, P O'Brien, B Durham, D Knaff, F Millett
Index: J. Biol. Chem. 262(23) , 11046-51, (1987)
Full Text: HTML
Abstract
In order to define the interaction domain on Rhodospirillum rubrum cytochrome c2 for the photosynthetic reaction center, positively charged lysine amino groups on cytochrome c2 were modified to form negatively charged carboxydinitrophenyl lysines. The reaction mixture was separated into six different fractions by ion exchange chromatography on carboxymethylcellulose and sulfopropyl-Sepharose. Peptide mapping studies indicated that fraction A consisted of a mixture of singly labeled derivatives modified at lysines 58, 81, and 109 on the back of cytochrome c2. Fractions C1, C2, C3, and C4 were found to be mixtures of singly labeled derivatives modified at lysines 9, 13, 75, 86, and 88 on the front of cytochrome c2 surrounding the heme crevice. The photooxidation of the carboxydinitrophenyl-cytochrome c2 derivatives by reaction centers purified from R. rubrum was measured following excitation with a laser pulse. The second-order rate constant of fraction A modified at backside lysines was found to be 2.3 X 10(7) M-1 s-1, nearly the same as that of native cytochrome c2, 2.6 X 10(7) M-1 s-1. However, the rate constants of fractions C1-C4 were found to be 6 to 12-fold smaller than that of native cytochrome c2. These results indicate that lysines surrounding the heme crevice of cytochrome c2 are involved in electrostatic interactions with carboxylate groups at the binding site of the reaction center. The reaction rates of horse heart cytochrome c derivatives modified at single lysine amino groups with trifluoroacetyl or trifluoromethylphenylcarbamoyl were also measured. Modification of lysines 8, 13, 25, 27, 72, 79, or 87 surrounding the heme crevice was found to significantly lower the rate of reaction, while modification of lysines in other regions had no effect. This indicates that the reaction of horse heart cytochrome c with the reaction center also involves the heme crevice domain.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
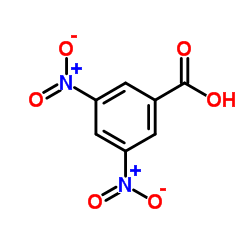 |
3,5-Dinitrobenzoic acid
CAS:99-34-3 |
C7H4N2O6 |
|
A method for rapid screening of ketone biotransformations: d...
2012-02-10 [Enzyme Microb. Technol. 50(2) , 101-6, (2012)] |
|
Electronic, infrared, and 1HNMR spectral studies of the nove...
2006-06-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 64(3) , 778-88, (2006)] |
|
Method development in quantitative NMR towards metrologicall...
2015-04-01 [Anal. Bioanal. Chem 407 , 3115-3123, (2015)] |
|
Using high-performance ¹H NMR (HP-qNMR®) for the certificati...
2014-05-01 [J. Pharm. Biomed. Anal. 93 , 102-110, (2014)] |
|
Synthesis, spectrophotometric, structural and thermal studie...
2010-08-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 76(3-4) , 315-21, (2010)] |