Convenient synthesis of pi-acceptor chiral stationary phases for high-performance liquid chromatography from halogen-substituted 3,5-dinitrobenzoylamides.
O R Malyshev, M G Vinogradov
Index: J. Chromatogr. A. 859(2) , 143-51, (1999)
Full Text: HTML
Abstract
A convenient method for the "in column" synthesis of chiral stationary phases for high-performance liquid chromatography was elaborated. It involves preparation of chiral amides of 2-bromo- or 4-chloro-substituted 3,5-dinitrobenzoic acids followed by nucleophilic substitution of the halogen in the aromatic moiety with 3-aminopropyl groups of silanized silica gel at ambient temperature. A series of pi-donor compounds, such as amides and alkyl aryl carbinols, were chromatographed on the prepared chiral stationary phases. The results were compared with data reported for chiral separations of the same substrates on similar (R)-N-(3,5-dinitrobenzoyl)-alpha-phenylglycine-derived CSP. An example of indirect enantioseparation of racemic alpha-phenylethylamine was also described using (R)-2-(2-bromo-3,5-dinitrobenzoylamino)-2-phenylethanol as a chiral derivatizing reagent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
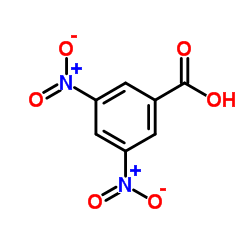 |
3,5-Dinitrobenzoic acid
CAS:99-34-3 |
C7H4N2O6 |
|
A method for rapid screening of ketone biotransformations: d...
2012-02-10 [Enzyme Microb. Technol. 50(2) , 101-6, (2012)] |
|
Electronic, infrared, and 1HNMR spectral studies of the nove...
2006-06-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 64(3) , 778-88, (2006)] |
|
Method development in quantitative NMR towards metrologicall...
2015-04-01 [Anal. Bioanal. Chem 407 , 3115-3123, (2015)] |
|
Using high-performance ¹H NMR (HP-qNMR®) for the certificati...
2014-05-01 [J. Pharm. Biomed. Anal. 93 , 102-110, (2014)] |
|
Synthesis, spectrophotometric, structural and thermal studie...
2010-08-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 76(3-4) , 315-21, (2010)] |