Indirect resolution of enantiomers of penicillamine by TLC and HPLC using Marfey's reagent and its variants.
R Bhushan, H Brückner, Virender Kumar
Index: Biomed. Chromatogr. 21(10) , 1064-8, (2007)
Full Text: HTML
Abstract
TLC and HPLC methods were developed for indirect chiral separation of penicillamine (3,3-dimethylcysteine) enantiomers after derivatization with Marfey's reagent (FDNP-Ala-NH(2)) and two of its structural variants, FDNP-Phe-NH(2) and FDNP-Val-NH(2). The binary mobile phase of phenol-water (3:1 v/v) and solvent combinations of acetonitrile and triethylamine phosphate buffer were found to give the best separation in normal and reversed-phase TLC, respectively. The diastereomers were also resolved on a reversed-phase C18 HPLC column with gradient elution of acetonitrile and 0.01 m trifluoroacetic acid. The results due to these three reagents were compared. The method was successful for checking the enantiomeric impurity of l-penicillamine in d-penicillamine and to check the enantiomeric purity of pharmaceutical formulations of d-penicillamine. The method was validated for linearity, repeatability, limit of detection and limit of quantification.2007 John Wiley & Sons, Ltd
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
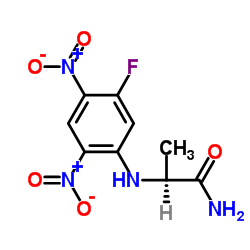 |
N2-(5-Fluoro-2,4-dinitrophenyl)-L-alaninamide
CAS:95713-52-3 |
C9H9FN4O5 |
|
Structural analysis reveals the substrate-binding mechanism ...
2015-04-13 [ChemBioChem. 16(6) , 924-9, (2015)] |
|
Biosynthesis of the new broad-spectrum lipopeptide antibioti...
2014-04-01 [Res. Microbiol. 165(3) , 243-51, (2014)] |
|
Structure elucidation and biosynthesis of fuscachelins, pept...
2008-10-07 [Proc. Natl. Acad. Sci. U. S. A. 105 , 15311-6, (2008)] |
|
Use of Marfey's reagent and analogs for chiral amino acid an...
2011-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(29) , 3148-61, (2011)] |
|
Chromatographic separation of enantiomers of non-protein alp...
2009-03-01 [Amino Acids 36(3) , 571-9, (2009)] |