| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
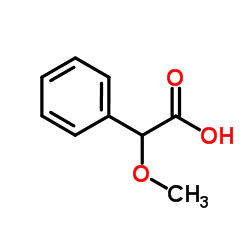 |
Methoxy(phenyl)acetic acid
CAS:7021-09-2 |
|
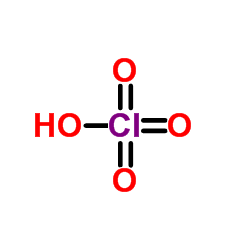 |
PERCHLORIC ACID
CAS:7601-90-3 |
|
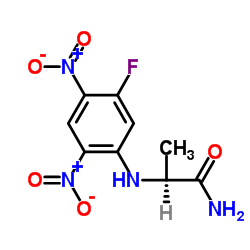 |
N2-(5-Fluoro-2,4-dinitrophenyl)-L-alaninamide
CAS:95713-52-3 |
|
 |
DL-Phenylalanine
CAS:150-30-1 |
|
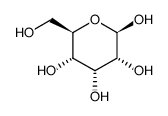 |
Beta-D-allose
CAS:7283-09-2 |