Enantiomeric determination of amphetamine and methamphetamine in urine by precolumn derivatization with Marfey's reagent and HPLC.
B S Foster, D D Gilbert, A Hutchaleelaha, M Mayersohn
Index: J. Anal. Toxicol. 22(4) , 265-9, (1998)
Full Text: HTML
Abstract
An analytical method was developed for enantiomeric determination of amphetamine and methamphetamine in human urine. The enantiomers were isolated from urine by solid-phase extraction, and diastereomers were formed by derivatization with the chiral Marfey's reagent (1-fluoro-2,4-dinitrophenyl-5-l-aniline amide). The diastereomers were separated by reversed-phase high-performance liquid chromatography in a water/methanol mobile phase and detected by absorbance spectrophotometry at 340 nm. Linear standard curves were obtained for all four enantiomers over a concentration range of 0.16-1.00 mg/L in urine. The detection limit was 0.16 mg/L urine for each enantiomer, and the limit of quantitation was 0.40 mg/L. The urine of 10 decedents was analyzed by this method and by a previously published precolumn derivatization procedure using (-)-1-(9-fluorenyl)ethyl chloroformate (FLEC) as the derivatizing agent and fluorescence detection. Comparison of the results of the two methods by linear regression showed comparable results for both d-amphetamine and d-methamphetamine. Neither method detected the presence of the l-enantiomers in the urine samples.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
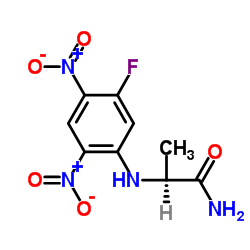 |
N2-(5-Fluoro-2,4-dinitrophenyl)-L-alaninamide
CAS:95713-52-3 |
C9H9FN4O5 |
|
Structural analysis reveals the substrate-binding mechanism ...
2015-04-13 [ChemBioChem. 16(6) , 924-9, (2015)] |
|
Biosynthesis of the new broad-spectrum lipopeptide antibioti...
2014-04-01 [Res. Microbiol. 165(3) , 243-51, (2014)] |
|
Structure elucidation and biosynthesis of fuscachelins, pept...
2008-10-07 [Proc. Natl. Acad. Sci. U. S. A. 105 , 15311-6, (2008)] |
|
Use of Marfey's reagent and analogs for chiral amino acid an...
2011-11-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(29) , 3148-61, (2011)] |
|
Chromatographic separation of enantiomers of non-protein alp...
2009-03-01 [Amino Acids 36(3) , 571-9, (2009)] |