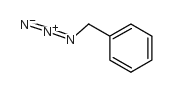
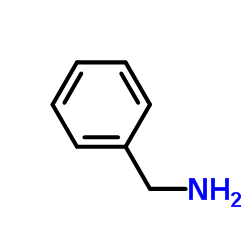
|
~79% |
|
~99% |
|
~89% |
|
~92% |
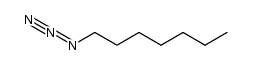

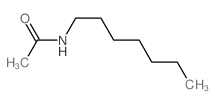
![(2S,4R)-2-(azidomethyl)-1-(tert-butoxycarbonyl)-4-[(methylsulfonyl)oxy]pyrrolidine Structure](https://image.chemsrc.com/caspic/298/113451-54-0.png)
![tert-Butyl (1S,4S)-2,5-diazabicyclo[2.2.1]heptan-2-carboxylate Structure](https://image.chemsrc.com/caspic/108/113451-59-5.png)